Selected recent reviews
Protein kinase Cλ/ι in cancer: a contextual balance of time and signals
Moscat J, Linares JF, Duran A, Diaz-Meco MT
Trends Cell Biol. 2022 Apr 29:S0962-8924(22)00090-3. doi: 10.1016/j.tcb.2022.04.002. Epub ahead of print. PMID: 35501226.
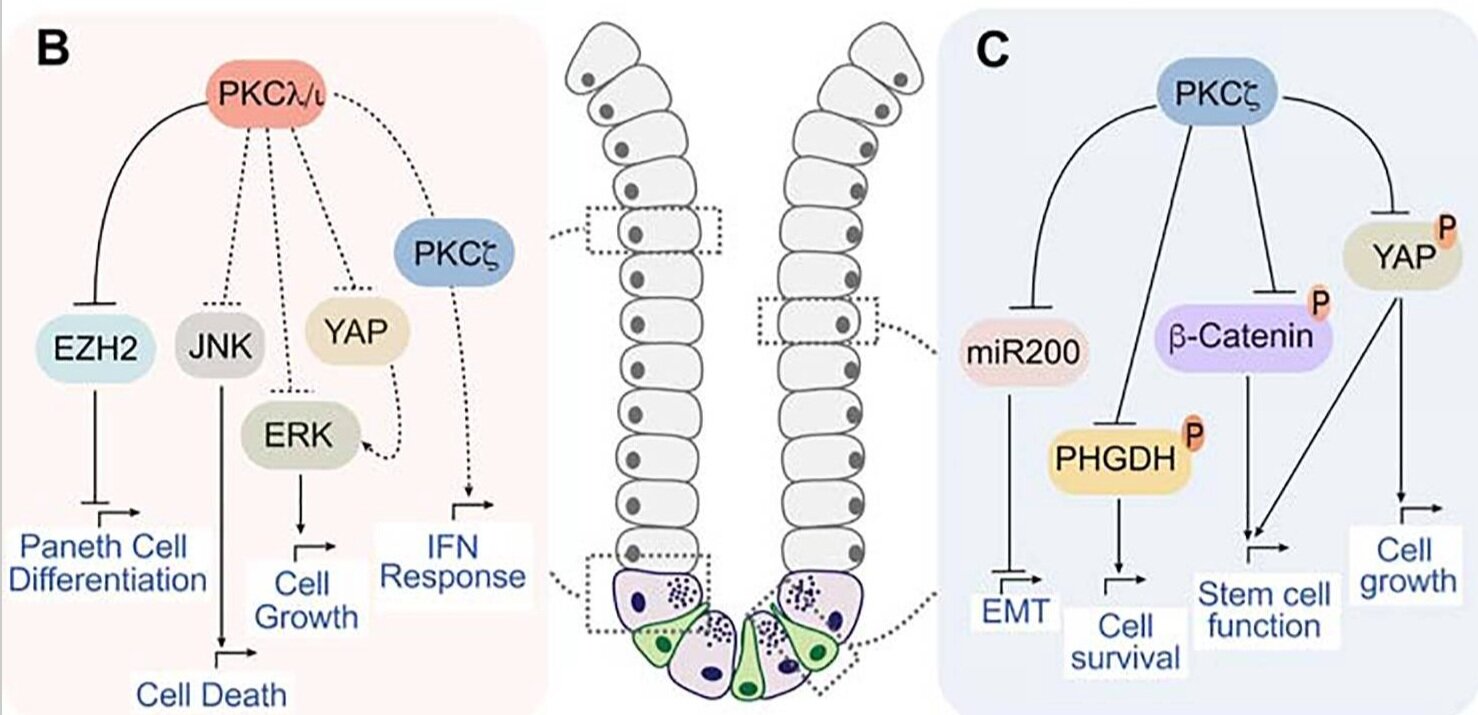
PKCλ/ι regulates epithelial cell lineage differentiation in the intestine and the prostate through epigenetic mechanisms, which are enabled, at least in prostate cancer cells, by the upregulation of ATF4, a critical step in the serine biosynthetic pathway for the generation of S-adenosyl-methionine, the obligate universal donor of methyl groups.
ATF4 is transcriptionally activated by mTORC1 through the PKCλ/ι-induced phosphorylation of LAMTOR2 at serine-30, which governs the aggregation of lysosomes and increases the amount of mTOR and RagC proximal to the nucleus, where RheB activates mTOR.
PKCλ/ι, in complex con p62, inhibits autophagy by directly phosphorylating LC3 at serine-12, while PKCλ/ι inactivation renders cells with increased autophagy activity that promotes oxidative phosphorylation triggered by PPARα and serves to eliminate ROS-damaged mitochondria, maintaining ROS levels below toxic concentrations, which enables the accumulation of NRF2 to activate tumorigenesis.
The loss of PKCλ/ι results in the activation of a new ULK2–TBK1 cascade that further reinforces the activation of autophagy to prevent the degradation of STING that helps stimulate the interferon pathway to promote CD8+ T cell recruitment and activation that inhibits tumor initiation and progression.
Nononcogenic cancer drivers often impinge on complex signals that create new addictions and vulnerabilities. Protein kinase Cλ/ι (PKCλ/ι) suppresses tumorigenesis by blocking metabolic pathways that regulate fuel oxidation and create building blocks for the epigenetic control of cell differentiation. Reduced levels of PKCλ/ι unleash these pathways to promote tumorigenesis, but the simultaneous activation of the STING-driven interferon cascade prevents tumor initiation by triggering immunosurveillance mechanisms. However, depending on the context of other signaling pathways, such as WNT/β-catenin or PKCζ, and timing, PKCλ/ι deletion can promote or inhibit tumorigenesis. In this review, we discuss in detail the molecular and cellular underpinnings of PKCλ/ι functions in cancer with the perspective of the crosstalk between metabolism and inflammation in the tumor microenvironment.
The Dual Roles of the Atypical Protein Kinase Cs in Cancer.
Reina-Campos M, Diaz-Meco MT, Moscat J.
Cancer Cell. 2019 Sep 16;36(3):218-235. doi: 10.1016/j.ccell.2019.07.010. Epub 2019 Aug 29.PMID: 31474570 Free PMC article. Review.
Atypical protein kinase C (aPKC) isozymes, PKCλ/ι and PKCζ, are now considered fundamental regulators of tumorigenesis. However, the specific separation of functions that determine their different roles in cancer is still being unraveled. Both aPKCs have pleiotropic context-dependent functions that can translate into tumor-promoter or -suppressive functions. Here, we review early and more recent literature to discuss how the different tumor types, and their microenvironments, might account for the selective signaling of each aPKC isotype. This is of clinical relevance because a better understanding of the roles of these kinases is essential for the design of new anti-cancer treatments.
The complexity of the serine glycine one-carbon pathway in cancer.
Reina-Campos M, Diaz-Meco MT, Moscat J.
J Cell Biol. 2020 Jan 6;219(1):e201907022. doi: 10.1083/jcb.201907022. PMID: 31690618
The serine glycine and one-carbon pathway (SGOCP) is a crucially important metabolic network for tumorigenesis, of unanticipated complexity, and with implications in the clinic. Solving how this network is regulated is key to understanding the underlying mechanisms of tumor heterogeneity and therapy resistance. Here, we review its role in cancer by focusing on key enzymes with tumor-promoting functions and important products of the SGOCP that are of physiological relevance for tumorigenesis. We discuss the regulatory mechanisms that coordinate the metabolic flux through the SGOCP and their deregulation, as well as how the actions of this metabolic network affect other cells in the tumor microenvironment, including endothelial and immune cells.
Serrated Colorectal Cancer: The Road Less Travelled?
Nakanishi Y, Diaz-Meco MT, Moscat J.
Trends Cancer. 2019 Nov;5(11):742-754. doi: 10.1016/j.trecan.2019.09.004. Epub 2019 Oct 28.PMID: 31735291 Free PMC article. Review.
Studies of colorectal cancer (CRC) originating through the conventional adenoma-carcinoma sequence have provided insight into the molecular mechanisms controlling its initiation and progression. Less is known about the alternative 'serrated' pathway, which has been associated with BRAF mutation and microsatellite instability. Recent transcriptomics approaches to classify human CRC revealed that mesenchymal and/or desmoplastic features combined with an immunosuppressive microenvironment are key determinants of CRC with the poorest prognosis. Importantly, these aggressive CRCs harbor the characteristics of serrated tumors, suggesting that initiation through this alternative pathway determines how aggressive the CRC becomes. Here, we review recent evidence on how serrated carcinogenesis contributes to the subtype of CRC with the poorest prognosis.
p62 in Cancer: Signaling Adaptor Beyond Autophagy.
Moscat J, Karin M, Diaz-Meco MT.
Cell. 2016 Oct 20;167(3):606-609. doi: 10.1016/j.cell.2016.09.030. PMID: 27768885 Free PMC article. Review.
Adaptor proteins participate in selective autophagy, which is critical for cellular detoxification and stress relief. However, new evidence supports an autophagy-independent key role of the adaptor p62 (encoded by the gene Sqstm1) in signaling functions central to tumor initiation in the epithelium and suppression of tumor progression in the stroma.
Feedback on fat: p62-mTORC1-autophagy connections.
Moscat J, Diaz-Meco MT.
Cell. 2011 Nov 11;147(4):724-7. doi: 10.1016/j.cell.2011.10.021.PMID: 22078874 Free PMC article. Review.
Metabolic homeostasis requires integration of multiple signals and cellular activities. Without this integration, conditions of obesity and diabetes often develop. Recent in vivo studies explore the molecular basis for metabolic homestasis, showing that p62 links autophagy and mTORC1 activation to regulate adipogenesis and energy control.
p62 at the crossroads of autophagy, apoptosis, and cancer.
Moscat J, Diaz-Meco MT.
Cell. 2009 Jun 12;137(6):1001-4. doi: 10.1016/j.cell.2009.05.023.PMID: 19524504 Free PMC article. Review.
The signaling adaptor p62 is a multidomain protein implicated in the activation of the transcription factor NF-kappaB. Recent findings link p62 activity to the extrinsic apoptosis pathway, and Mathew et al. (2009) now show that the modulation of p62 by autophagy is a key factor in tumorigenesis. These findings place p62 at critical decision points that control cell death and survival.
Cell signaling and function organized by PB1 domain interactions.
Moscat J, Diaz-Meco MT, Albert A, Campuzano S.
Mol Cell. 2006 Sep 1;23(5):631-40. doi: 10.1016/j.molcel.2006.08.002.PMID: 16949360 Review.
The PB1-domain-containing proteins p62, aPKC, MEKK2/MEKK3, MEK5, and Par-6 play roles in critical cell processes like osteoclastogenesis, angiogenesis, and early cardiovascular development or cell polarity. PB1 domains are scaffold modules that adopt the topology of ubiquitin-like beta-grasp folds that interact with each other in a front-to-back mode to arrange heterodimers or homo-oligomers. The different PB1 domain adaptors provide specificity for PB1 kinases to ensure the effective transmission of cellular signals. Also, recent data suggest that PB1 domains may serve to orchestrate signaling cascades not involving other PB1 domains, such as the MEK5-ERK5 and p62-ERK1 interactions.
Metabolic reprogramming of the tumor microenvironment by p62 and its partners.
Reina-Campos M, Shelton PM, Diaz-Meco MT, Moscat J.
Biochim Biophys Acta Rev Cancer. 2018 Aug;1870(1):88-95. doi: 10.1016/j.bbcan.2018.04.010. Epub 2018 Apr 25.PMID: 29702207 Free PMC article. Review.
The concerted metabolic reprogramming across cancer and normal cellular compartments of the tumor microenvironment can favor tumorigenesis by increasing the survival and proliferating capacities of transformed cells. p62 has emerged as a critical signaling adaptor, beyond its role in autophagy, by playing an intricate context-dependent role in metabolic reprogramming of the cell types of the tumor and stroma, which shapes the tumor microenvironment to control tumor progression. Focusing on metabolic adaptations, we review the cellular processes upstream and downstream of p62 that regulate how distinct cell types adapt to the challenging and evolving environmental conditions during tumor initiation and progression. In addition, we describe partners of p62 that, in a collaborative or independent manner, can also rewire cell metabolism. Finally, we discuss the potential therapeutic implications of targeting p62 in cancer, considering its multifaceted roles in diverse cell types of the tumor microenvironment.
Metabolism shapes the tumor microenvironment.
Reina-Campos M, Moscat J, Diaz-Meco M.
Curr Opin Cell Biol. 2017 Oct;48:47-53. doi: 10.1016/j.ceb.2017.05.006. Epub 2017 Jun 9.PMID: 28605656 Free PMC article. Review.
Tumors are strongly influenced by the surrounding normal tissue, which forms a specialized niche termed the tumor microenvironment (TME). The TME is modeled by cancer cells for their own benefit through a complex array of interactions. The identification of new forms of communication within the TME, which are dependent on the tumor's metabolic activity, has expanded our understanding of this heterocellular regulation and has revealed potential therapeutic targets. This review will summarize recent findings on the metabolic regulation of tumor cells by the TME. The concepts to be discussed include the existence of metabolic intratumoral heterogeneity, the contribution of cancer associated fibroblasts (CAFs) to tumor progression, and the regulation of tumor immunology by tumor-secreted metabolites.