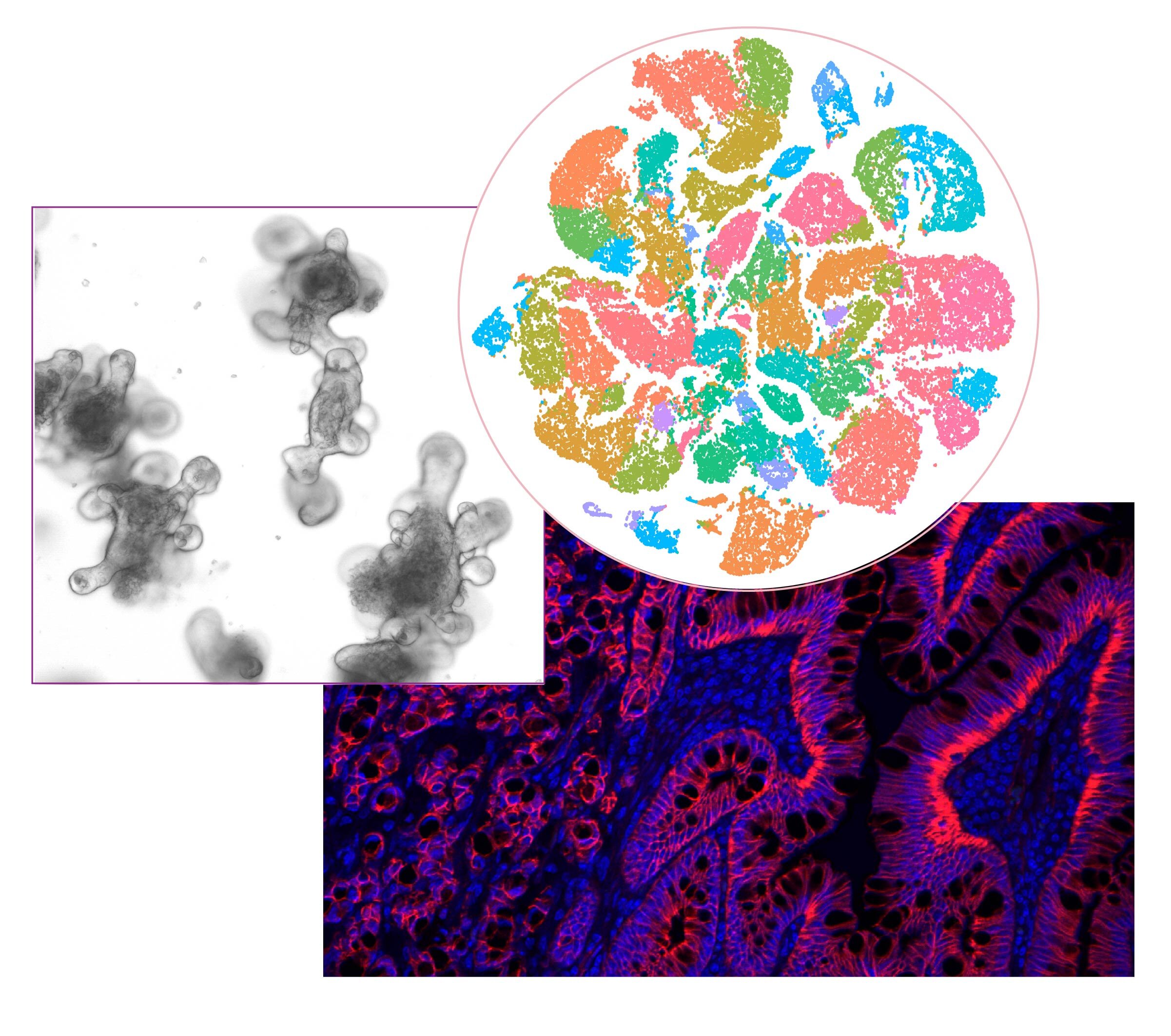
Understanding how colorectal cancer initiates and progresses
An emerging notion in the colorectal cancer (CRC) field is that a stem-mesenchymal phenotype of the tumor epithelium, together with an immunosuppressive and desmoplastic stroma, predicts adverse outcomes in CRC patients better than the presence of prevalent mutations. Although mutations in critical proto-oncogenic and tumor suppressor genes drive the initial phases of CRC development, however, they only have a limited value for the prediction of heterogeneity and therapy resistance, metastatic potential, and poorly informs the therapeutic strategy and is quite limited for the prediction of patient survival. Although transcriptomics better classifies the different types of CRC, the molecular and cellular mechanisms driving disease progression and the role of the microenvironment in that process still needs to be fully understood. Our laboratories have identified the PB1-containing kinases PKCz and PKCl/i, as novel tumor suppressors acting in concert to prevent the most aggressive forms of CRC through unique mechanisms that involve the reprogramming of the tumor epithelium to generate new forms of cancer stem cells, as well as to reprogram the tumor microenvironment. Our studies are aimed at identifying the precise signaling pathways that regulate the process of tumor transformation towards the aggressive mesenchymal stage and to establish new potential therapeutic targets for CRC treatment in an oncogenic-agnostic manner.
Kinoshita H, Martinez-Ordoñez A, Cid-Diaz T, Han Q, Duran A, Muta Y, Zhang X, Linares JF, Nakanishi Y, Kasashima H, Yashiro M, Maeda K, Albaladejo-Gonzalez A, Torres-Moreno D, Garcia-Solano J, Conesa-Zamora P, Inghirami G, Metallo CM, Diaz-Meco MT, Moscat J. (2024) Epithelial aPKC deficiency leads to stem cell loss preceding metaplasia in colorectal cancer initiation. Developmental Cell 59, 1–16. https://doi.org/10.1016/j.devcel.2024.05.001.
Muta Y, Linares JF, Martinez-Ordoñez A, Duran A, Cid-Diaz T, Kinoshita H, Zhang X, Han Q, Nakanishi Y, Nakanishi N, Cordes T, Arora GK, Ruiz-Martinez M, Reina-Campos M, Kasashima H, Yashiro M, Maeda K, Albaladejo-Gonzalez A, Torres-Moreno D, Garcia-Solano J, Conesa-Zamora P, Inghirami G, Metallo CM, Osborne TF, Diaz-Meco MT, Moscat J. (2023) Enhanced SREBP2-driven cholesterol biosynthesis by PKCλ/ι deficiency in intestinal epithelial cells promotes aggressive serrated tumorigenesis. Nat Commun 14, 8075. https://doi.org/10.1038/s41467-023-43690-5. PMID: 38092754
Martinez-Ordoñez A, Duran A, Ruiz-Martinez M, Cid-Diaz T, Zhang X, Han Q, Kinoshita H, Muta Y, Linares JF, Kasashima Y, Omar M, Nishimura S, Avila L, Yashiro M, Maeda K, Pannellini T, Pigazzi A, Inghirami G, Marchionni L, Sigal D, Diaz-Meco MT, Moscat J. (2023) Hyaluronan driven by epithelial aPKC deficiency remodels the microenvironment and creates a vulnerability in mesenchymal colorectal cancer. Cancer Cell. Feb 13, 41 Feb 13;41(2):252-271.e9. doi: 10.1016/j.ccell.2022.11.016. Epub 2022 Dec 15. PMID: 36525970.
Linares JF, Zhang X, Martinez-Ordoñez A, Duran A, Kinoshita H, Kasashima H, Nakanishi N, Nakanishi Y, Carelli R, Cappelli L, Arias E, Yashiro M, Ohira M, Patel S, Inghirami G, Loda M, Cuervo AM, Diaz-Meco MT, Moscat J. (2021) PKCλ/ι inhibition activates an ULK2-mediated interferon response to repress tumorigenesis. Molecular Cell. 81, 4509-4526.e10. PMID: 34560002
Nakanishi, Y., Duran, A., L’Hermitte, A., Shelton, P.M., Nakanishi, N., Reina-Campos, M., Huang, J., Soldevila,F., Baaten,B.J.G., Tauriello, D.V.F., Castilla, E.A., Bhangoo, M.S., Bao, F., Sigal, D., Diaz-Meco, M.T., Moscat, J. (2018) Simultaneous Loss of both Atypical Protein Kinase C Genes in the Intestinal Epithelium Drives Serrated Intestinal Cancer by Impairing Immunosurveillance. Immunity 49, 1132–1147. PMC6301096
Shelton, P.M., Duran, A., Nakanishi, Y., Reina-Campos, M., Kasashima, H., Llado, V., Ma, L., Campos, A., Garcia-Olmo, D., Garcia-Arranz, M., Garcia-Olmo, D.C., Olmedillas-Lopez, S., Caceres, J.F., Diaz-Meco, M.T., Moscat, J. (2018) The Secretion of miR-200s by a PKCz/ADAR2 Signaling Axis Promotes Liver Metastasis in Colorectal Cancer. Cell Reports. 23(4):1178-1191. PMC5958623.
Nakanishi, Y., Reina-Campos, M., Nakanishi, N., Llado, V., Elmen, L., Peterson, S., Campos, A., De, S.K., Leitges, M., Ikeuchi, H., Pellecchia, M., Blumberg, R.S., Diaz-Meco, M.T., Moscat, J. (2016) Control of Paneth Cell Fate, Intestinal Inflammation, and Tumorigenesis by PKCl/i. Cell Reports 16, 3297-3310 PMID: 27653691
Llado, V., Nakanishi, Y., Duran, A., Reina-Campos, M., Shelton, P.M., Linares, J.F., Yajima, T., Campos, A., Aza-Blanc, P., Leitges, M., Diaz-Meco, M.T., Moscat, J. (2015) Repression of Intestinal Stem Cell Function and Tumorigenesis through Direct Phosphorylation of b-Catenin and Yap by PKCz. Cell Reports 10, 740-754. PMID: 25660024
Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA, Chen J, Yajima T, Porollo A, Medvedovic M, Brill LM, Plas DR, Riedl SJ, Leitges M, Diaz-Meco MT, Richardson AD, Moscat J. (2013) Control of nutrient stress-induced metabolic reprogramming by PKCζ in tumorigenesis. Cell 152, 599-611. PMID: 23374352